electron configuration for na+|Sodium Electron Configuration (Na) with Orbital Diagram : Tuguegarao Mar 23, 2023 Resultado da ui. 1. 2K votes, 55 comments. 120K subscribers in the BrasiIeirasGostosas community. sub dedicado a todas gostosas do Brasil, seja ela famosa ou não! 🇧🇷.
PH0 · What is the electron configuration of Na?
PH1 · Sodium Electron Configuration (Na) with Orbital Diagram
PH2 · Sodium Electron Configuration
PH3 · Sodium (Na): How to write the Orbital Diagram, Electron
PH4 · How to Write the Electron Configuration for Sodium (Na)
PH5 · Electron Configuration Chart of All Elements (Full Chart)
PH6 · Electron Configuration Calculator
PH7 · 3.1: Electron Configurations
PH8 · 2.7: Electron Configurations
PH9 · 2.4 Electron Configurations
WEBAcesse a internet para consultar seus exames no Hospital Universitário de Brasília, uma instituição de saúde pública localizada em Brasília. Você precisa digitar a chave de .
electron configuration for na+*******In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom.
In order to write the Mg electron configuration we first need to know the .
How to Write the Electron Configuration for Neon. Neon is the tenth element with a . Mar 23, 2023 In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the .
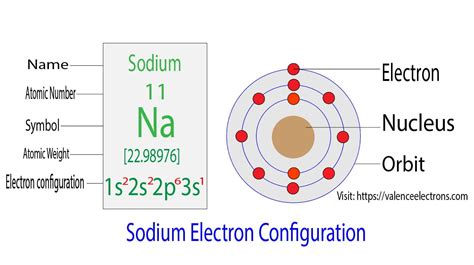
This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl. The unabbreviated electron configuration for Na can be represented as: 1s22s22p63s1. How Do You Find The Electron Configuration For Sodium? To find the electron configuration firstly you .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .electron configuration for na+ Sodium Electron Configuration (Na) with Orbital Diagram This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at .
Learning Objectives. Describe how electrons are arranged in an atom using electron configurations. Previously we discussed the concept of electron shells, subshells, orbitals, and electron spin. It is the .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . The electrons fill up according to the rules from the lowest energy level first, filling available s and p orbitals, with a maximum number of 2 electrons per orbital with .
In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom.
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.
In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all. This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl. The unabbreviated electron configuration for Na can be represented as: 1s22s22p63s1. How Do You Find The Electron Configuration For Sodium? To find the electron configuration firstly you should be aware of the number of electron in the element. The sodium has 11 numbers of electrons.
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.Learning Objectives. Describe how electrons are arranged in an atom using electron configurations. Previously we discussed the concept of electron shells, subshells, orbitals, and electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . The electrons fill up according to the rules from the lowest energy level first, filling available s and p orbitals, with a maximum number of 2 electrons per orbital with opposite spins.In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.
electron configuration for na+ In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we'll put all.Sodium Electron Configuration (Na) with Orbital Diagram This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl.
The unabbreviated electron configuration for Na can be represented as: 1s22s22p63s1. How Do You Find The Electron Configuration For Sodium? To find the electron configuration firstly you should be aware of the number of electron in the element. The sodium has 11 numbers of electrons.
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table.

This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
Learning Objectives. Describe how electrons are arranged in an atom using electron configurations. Previously we discussed the concept of electron shells, subshells, orbitals, and electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
Baixe e use 80.000+ fotos profissionais de Soccer Wallpaper gratuitamente. Milhares de novas imagens todos os dias Uso completamente gratuito Vídeos e imagens de alta .
electron configuration for na+|Sodium Electron Configuration (Na) with Orbital Diagram